Abstract
Tyrosine kinase inhibitors (TKIs) have significantly improved the prognosis of patients with chronic myeloid leukemia (CML). Dasatinib, one of the most utilized TKIs, is formulated as a crystalline dasatinib monohydrate. This results in a low and pH-dependent solubility with highly variable intestinal absorption and plasma pharmacokinetics (PK). Poor dissolution of an anti-cancer drug is generally expected to result in low and highly variable bioavailability and plasma exposure, consequently jeopardizing an optimal and anticipated therapeutic response. Applying bioavailability enhancement techniques, such as amorphous solid dispersion (ASD) formulations, will reduce variability in plasma PK as solubility and intestinal absorption improves, and most importantly increase patient compliance, safety, and efficacy.
XS004 dasatinib (XS004) (Xspray Pharma, Solna, Sweden) is a novel immediate release and ASD formulation of dasatinib produced with the HyNap™ technique. XS004 is designed to provide an increased solubility of dasatinib in acidic to neutral pH conditions, mitigating gastric pH dependency and leading to reduced variability and increased absorption and bioavailability of dasatinib.
Here we report the results of a clinical study comparing the PK of XS004 and the original dasatinib product (reference) (Sprycel®, Bristol-Myers Squibb Company, NJ, USA), and results of a comparative in vitro solubility study. Plasma PK parameters were compared following single doses of XS004 (100 mg dasatinib ASD) and the reference product (140 mg dasatinib non-ASD) in 107 healthy volunteers at fasting conditions in a randomized, single dose, laboratory-blinded, 2 treatments, 4 period, 2 sequence, full replicate crossover, bioavailability study. In the in vitro study, dasatinib ASD and dasatinib monohydrate solubility was determined in the gastrointestinal (GI) relevance pH-interval of 1.2 to 8.0 at 37°C.
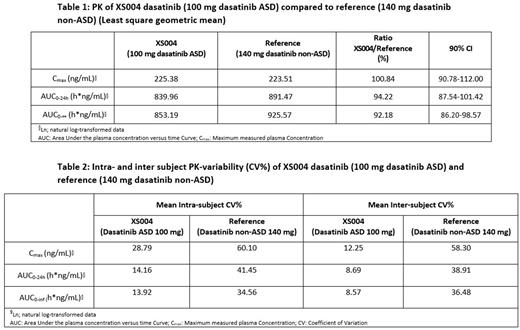
The plasma exposure of XS004 (100 mg dasatinib ASD) was equivalent for all PK parameters with the reference product (140 mg dasatinib non-ASD) with least square geometric mean Cmax, AUC0-24h and AUC0-inf ratios of 100.8 (CI 90%, 90.8-112.0%), 94.2 (CI 90%, 87.5-101.4) and 92.2 (CI 90%, 86.2-98.6), respectively (Table 1).
The intra-subject variability (coefficient of variation (CV%)) for AUC0-24 and AUC0-inf for the reference was approximately 3-fold and 2.5-fold higher than for XS004 (Table 2). For Cmax, the intra-subject variability was approximately 2-fold greater with the reference compared to XS004 (Table 2).
The inter-individual variability was generally higher in all plasma PK parameters for the reference formulation (range: 38-128%) when compared to XS004 (range: 20-48%). There was considerably greater inter-subject variability (CV%) in Cmax, AUC0-24 and AUC0-inf after single dose administration of the reference product, when compared with XS004 (Table 2). The inter-individual variability was 4.5-fold, 4.3-fold and 4.8-fold greater for the reference product when compared to the test formulation for the three PK parameters.
The in vitro solubility study provided higher apparent solubility of dasatinib ASD compared to the original crystalline dasatinib, explaining the improved in vivo GI absorption for the new preparation. Dasatinib ASD was unaffected and kept in solution over at least 2 hours in the pH range 1.2 to 5.0 and the solubility was at least 10-fold higher for dasatinib ASD compared to the dasatinib monohydrate at pH-values in the range 5.0 to 8.0.
In conclusion, XS004 displayed a more robust and consistent GI-absorption and bioavailability, with significantly reduced intra- and inter-individual variability in plasma dasatinib concentrations, compared to the original non-ASD formulated dasatinib. The in vitro data showed that the solubility of dasatinib ASD was substantially less pH-dependent at GI-relevant pH values of 2.0 to 5.0.
The results suggest that XS004, an ASD formulation of dasatinib, with improved biopharmaceutical features, reduces the risk of both low exposure and high exposure outliers, with lower risk of exposure outside the therapeutic window. This would likely diminish the risk of adverse events and optimize the therapeutic efficacy of dasatinib in patients with CML.
Disclosures
Lennernäs:Xspray Pharma: Consultancy, Current equity holder in publicly-traded company. Liljebris:Xspray Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Current holder of stock options in a publicly-traded company. Brisander:Xspray Pharma: Consultancy, Current equity holder in publicly-traded company, Other: Current holder of stock options in a publicly traded company. Jesson:Xspray Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Current holder of stock options in a publicly traded company. Andersson:Xspray Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Current holder of stock options in a publicly traded company. Larfors:Xspray Pharma: Consultancy. Stenke:Xspray Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.